This finding is consistent with preterm births occurring throughout the world, with 15 million premature babies born worldwide each year (2). The prevalence of preterm births has steadily increased over the last two decades and has reached 11.5% of worldwide births. Most of this increase has been in infants born between 32 and 36 weeks gestation.
1. Martin JA, Hamilton BE, Osterman MJK, et al. “Births: Final data for 2013. National vital statistics reports”; Vol 64 No 1, Hyattsville, MD: National Center for Health Statistics (2015)
2. http://www.marchofdimes.org/mission/world¬prematurity-day.aspx
3. Richard E. Behrman, Adrienne Stith Butler, eds “Preterm birth: causes, consequences, and prevention / Committee on Understanding Premature Birth and Assuring Healthy Outcomes, Board on Health Sciences Policy”; Washington (DC): National Academies Press (US); 2007. The National Academies Collection: Reports funded by National Institutes of Health.
Pre-Term Nutrition Care
Compared with infants born at term, preterm infants are at greater risk of of complications, altered growth, death, and disability. Twenty percent of infants born before 32 weeks gestation and 1% of infants born between 32 and 36 weeks gestation do not survive the first year of life (4).
Although recent advances in medical technologies and Neonatal Intensive Care Unit (NICU) care have led to improved mortality rates, surviving infants are at significant risk of morbidity. The complications of preterm births arise from immature organ systems that are not yet prepared to support life in the extrauterine environment. These include pediatric diseases and physiological states at birth or shortly after birth, as well as diseases and conditions which develop at a later stage.
Main complications/diseases may include:
• Necrotizing Enterocolitis (NEC) (5)
• Short Bowel Syndrome (Short Gut Syndrome)
• Bronchopulmonary Dysplasia (BPD) (6)
• Adverse neurodevelopmental outcomes
Preventing and managing these complications involves nutritional management of preterm infants. which are often not optimal with negative effects on the body composition and these infants.
4. Preterm Birth Book.
5. Reynolds RM, Thureen PJ “Special Circumstances: Trophic feeds, necrotizing enterocolitis and bronchopulmonary dysplasia”; Seminars in Fetal & Neonatal Medicine (2007) 12, 64-70.
6. Huysman WA, de Riddler M de Bruin NC, van Helmond G, Terpstra N, Van Goudoever JB, Sauer PJJ “Growth and Body composition in preterm infants with bronchopulmonary dysplasia”; Archives of Disease in Childhood Fetal and Neonatal Edition 2003; 88:F46-F51.
Effect of Current Nutritional Practices on Body Composition and Adverse Long Term Effects Over the past decades, in addition to improvements in maternal care, technological advances in thermal management, respiratory care, and nutrition support of newborns have significantly improved the survival rates of preterm infants (3). Beyond initial survival, one of the primary concerns of preterm infants is their brain development. Current practices are tailored to providing nutrition of a high caloric value and aimed at maintaining in-utero rates of growth (changes in body weight), not quality of growth (changes in body composition). While this practice has shown success in improving neurodevelopmental outcomes, preterm infants have a higher % fat and lower fat-free mass at term adjusted age (corrected age) when compared with full-term infants (7-9). Higher % fat in preterm infants continues until 3 months corrected age, at which point preterm and term babies have similar adiposity levels (10, 11). Based on several epidemiological studies in preterm and term infants, the metabolic programming that occurs during this window of altered adiposity is responsible for higher levels of metabolic diseases (heart disease, stroke,
obesity, and diabetes) later in life (12, 13).
7. Giannì ML, Roggero P, Taroni F, Liotto N, Piemontese P, Mosca F. “Adiposity in small for gestational age preterm infants assessed at term equivalent age”; Arch Dis Child Fetal Neonatal Ed, 94(5):F368-372 (2009)
8. Olhager E, Törnqvist C. “Body composition in late preterm infants in the first 10 days of life and at full term”; Acta Paediatr, 103(7):737-743 (2014)
9. Simon L, Frondas-Chauty A, Senterre T, Flamant C, Darmaun D, Rozé JC. “Determinants of body composition in preterm infants at the time of hospital discharge”; Am J Clin Nutr, 100:98-104 (2014)
10. Giannì ML, Roggero P, Liotto N, Amato O, Piemontese P, Morniroli D, Bracco B, Mosca F. “Postnatal catch-up fat after late preterm birth”; Pediatr Res, 72(6):637-640 (2012)
11. Ramel SE, Gray HL, Ode KL, Younge N, Georgieff MK, Demerath EW. “Body composition changes in preterm infants following hospital discharge: comparison with term infants” J Pediatr Gastroenterol Nutr, 53(3):333-338 (2011)
12. Wells J. “Body composition in infants: evidence for developmental programming and techniques for measurement”; Rev Endocr Metab Disord, 13:93-101 (2012)
13. Lafeber HN, van de Lagemaat M, Rotteveel J, van Weissenbruch M. “Timing of nutritional interventions in very-lowbirth-weight infants: optimal neurodevelopment compared with the onset of the metabolic syndrome”; Am J Clin Nutr, Aug; 98(2):556S-60S (2013)
New Findings in Pre-Term Nutrition Care
Better evidence and understanding of preterm birth, brain development, nutrition, and post-natal changes in body composition (and their effect on metabolic health later in life) have resulted in a re-examining of the links among brain development, nutrition, and metabolic health. Historically, it was assumed that brain development and metabolic health were mutually exclusive. Therefore, neonatologists focused on interventions, including nutritional regiments, aimed at brain health and development without paying much attention to changes in body
composition. Recently, this thinking has begun to shift. It is now recognized that brain development might be directly, and not inversely, linked to body composition.
Recent studies in preterm infants show that brain development and processing at 4 and 12 months of age are associated with increased fat-free mass gains but not fat mass gains (14, 15). These studies and others (16, 17) have pointed to the importance of measuring and tracking body composition as better indicators of healthy growth, and have shown that changes in fat and fat-free mass affect brain and metabolic development in similar ways (rapid body composition changes and significant deviations from in-utero growth are not beneficial) without the two being mutually exclusive. These studies also provide recommendations for protein and amino acids supplementation shown to achieve both positive overall growth and appropriate quality of growth (fat and fatfree mass changes) with positive consequences for both brain and metabolic development.
14. Pfister KM, Gray HL, Miller NC, Demerath EW, Georgieff MK, Ramel SE. “Exploratory study of the relationship of fat-free mass to speed of brain processing in preterm infants”; Pediatr Res, 74(5):576-583 (2013)
15. Ramel SE, Gray HL, Christiansen E, Boys C, Georgieff MK, Demerath EW. “Greater early gains in fat-free mass, but not fat mass, are associated with improved neurodevelopment at 1 year corrected age for prematurity in very low birth weight preterm infants”; J Pediatr, 173:108-115 (2016)
16. Amesz EM, Schaafsma A, Cranendonk A, Lafeber HN. “Optimal growth and lower fat mass in preterm infants fed a
protein-enriched postdischarge formula”; J Pediatr Gastroenterol Nutr, Feb;50(2):200-7 (2010)
17. Corpeleijn WE, Kouwenhoven SM, van Goudoever JB. “Optimal growth of preterm infants”; World Rev Nutr Diet, 106:149-155 (2013)
Future Directions
In the last decade, research studies have advanced our understanding of the complex interaction between preterm birth, brain development, nutrition, and the effects of post-natal changes in body composition on short and long-term health. It is now understood that in preterm infants, brain and metabolic health are not mutually exclusive, but rather connected and both benefitting from proper nutrition and tracking of body composition. This knowledge, and the use of new technologies such as breast milk analyzers, breast milk fortifiers, specialized formulas, and body composition assessment tools to track the effects of nutritional regiments, have resulted in a more individualized and integrated approach to the care of preterm infants. This emerging trend is being spearheaded by a number of clinical research centers around the world. As these practices become more accessible and understood, their eventual widespread implementation will lead to better short- and long-term outcomes for a growing number of preterm infants.
Clinical Applications
Some clinical uses of Infant Body Composition information include.
1. Nutritional intervention to ensure normal growth trajectory
Current feeding practices in the NICU target growth indices based on growth reference charts which do not include body composition information. It is also known that the quality of rapid growth is an important factor in the early postnatal period. A body composition reference curve, used along with existing growth charts such as the CDC NCHS, Babson Benda, and Fenton charts, will help nutritionists adjust the micro and macro nutrient content of nutrition provided.
During the course of disease in infants, energy needs are affected by the underlying disease and current nutritional status. Some diseases have been shown to increase or decrease nutritional needs (18). Body composition information can be used to determine the current nutritional status and will provide baseline information. Preterm infants recovering from pediatric disease states and complications like NEC, BPD, PDA, SBS, etc., have significantly altered body composition as a result of either malnutrition or alternate feeding therapies. During recovery it is important to calculate energy needs, as well as protein needs, to accrue new tissue to ensure growth (18). Body composition
data will be useful in calculating energy and protein requirements for these infants. Nutrition to preterm infants who are SGA provides for catch-up growth and tries to maintain in-utero rates of growth. Early aggressive nutrition, while achieving the above, may cause higher adiposity levels in infants. Infant body composition can be monitored to see the effect of nutrition provided. Adjusting the protein and caloric
content of nutrition provided to these infants based on body composition will ensure optimal growth without adding to the risk of metabolic syndrome in later adult life.
18. Durnwald C, Huston-Presley L, Amini S, Catalano P. “Evaluation of body composition of large-for-gestationalage infants of women with gestational diabetes mellitus compared with women with normal glucose tolerance levels”; American Journal of Obstetrics & Gynecology, 2004 Vol. 191 No.3 804-808.
2. Use of body composition data to calculate dosage and treatment requirements
Current treatment requirements are tailored to body size and sometimes body surface area, as opposed to body composition. Examples include nutrition and fluid intake, drug dosages, radiation dosages, and dialysis. Pharmacological substances are ineffective at low concentrations and toxic at high concentrations (19). Most treatments and dosage requirements are currently based on body weight. The higher metabolic rate of infants is well recognized and, since over 99% of metabolic processes occur in lean mass, dosage calculations based on an infant’s lean mass would be a more appropriate criterion in administering hydrophilic drugs (20). Fat mass may also be relevant in calculating anesthetic doses. Some anesthetic drugs are fat-soluble and absorbed into adipose tissue by diffusion. In obese individuals, the subsequent release of the drug from adipose tissue can delay recovery (21). The effectiveness of anesthesia administration in the infant population using body composition data may improve clinical outcomes and needs more investigation (19).
19. Unpublished Data: Gilchrist JM. “Body Composition Reference Data for Exclusively Breast-Fed Infants”; Presented at Pediatric Academic Societies Annual Meeting, May 5-7, 2007.
20. Barker DJP, Eriksson JG, Forsen T, Osmond C. “Fetal origins of adult disease: strength of effects and biological basis”; Intl Journal of Epidemiology 2002 31, 1235-1239.
21. Wells JCK “The thrifty phenotype as an adaptive maternal effect”. Biological Reviews 2007 82, 143-172
3. Optimizing NICU release criteria
Current NICU release criteria include, but are not limited to the following:
- Consistent weight gain over a period of days
- Tolerating oral feedings
- Ability to maintain normal body temperature
While consistent weight gain over a period of days is currently seen as a discharge criterion, qualifying the weight gain will help in identifying the infant’s ability to maintain optimal body composition, and is even more important in preterm infants. Therefore, a better criterion would be to look at weight percentile together with body composition data.
In cases where the body composition of the infant deviates from the body composition reference curve, post discharge nutritional recommendations can be made to achieve optimal body composition, while providing for adequate growth.
4. Develop normative growth data using body composition
Developing term and preterm normative body composition to define how an infant is supposed to grow. This normative body composition data can be used to:
- Refine nutrition guidelines
- Assess quality of growth in infants
- Assess nutritional status in regions and countries
- Add to current growth reference charts
- Develop better infant formulas
Measurement Technologies
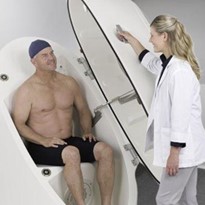
Densitometry (PEA POD)
This technique uses Air Displacement Plethysmography (ADP) via a device called the PEA POD®. An infant’s body composition (fat and fat-free mass) are calculated from body density (Density Body = Mass Body/Volume Body). It is a complete turnkey system using the same patented air displacement technology as the BOD POD® Body Composition Tracking System, which has been used successfully for assessing the body composition of children and adults since 1994. The PEA POD is extremely simple to operate, with software prompts guiding the operator through each step of the process. This includes inputting infant information into the software, weighing the infant, and measuring the infant’s body volume inside the PEA POD chamber. Testing is completely safe and noninvasive, and there are no compliance issues. The temperature-controlled test chamber provides a comfortable test environment for the infant, and a complete analysis takes about 5 minutes.
Validation of the PEA POD has been performed against the deuterium dilution method and a reference 4-compartment model for the estimation of infant body composition. It was found to be accurate and precise, with excellent within-day and between-day reliability. The ease of use, minimum safety concerns, and bedside accessibility, make the PEA POD highly suitable for monitoring changes in body composition during infant growth in research and clinical settings (27).
22. Forsum E, Olhager E and Törnqvist C “An Evaluation of the Pea Pod System for Assessing Body Composition of Moderately Premature Infants” Nutrients 2016, 8(4), 238
23. Ramel SE, Gray HL, Davern BA, Demerath EW “Body composition at birth in preterm infants between 30 and 36 weeks gestation” Pediatr Obes. 2015 Feb;10(1):45-51.
24. Fields DA, Gunatilake R, Kalaitzoglou E “Air Displacement Plethysmography: Cradle to Grave” Nutr Clin Pract. 2015 Mar 11.
25. Li C, McCargar LJ, Casey LM “Infant body composition in the PEA POD® era: what have we learned and where do we go from here?” J Dev Orig Health Dis. 2013 Apr;4(2):116-20.
26. Roggero P, Giannì ML, Amato O, Piemontese P, Morniroli D, Wong WW, Mosca F “Evaluation of air-displacement plethysmography for body composition assessment in preterm infants” Pediatr Res. 2012 Sep;72(3):316-20. doi:10.1038/pr.2012.75. Epub 2012 Jun 5.
27. Ellis KJ, Yao M, Shypailo RJ, Urlando A, Wong WW, Heird WC “Body-composition assessment in infancy: air-displacement plethysmography compared with a reference 4-compartment model” Am J Clin Nutr. 2007 Jan;85(1):90-5.
Total Body Water (TBW)
This technique is based on the nutrition model of body composition. Since Total Body Water (TBW) is the main component of Fat Free Mass (FFM), if body water can be measured and the ratio of TBW to FFM is known, then Fat Mass (FM) can be calculated. To measure body water directly, an infant is administered a small amount of water labeled with deuterium, a non-radioactive tracer. Urine samples are collected before the oral dose and after several hours of the dose. Samples are analyzed using isotope-ratio mass spectrometry. Repeat measurements can be done only after the tracer from a previous measurement has cleared from the body, which is typically 10-14 days for infants. The unavailability of the capability for mass spectrometry analysis in many labs, and the cumbersomeness of the procedure has not lead to widespread use of this technology.
28. Evaluation of body composition in neonates and infants. Ellis KJ. Seminars in Fetal and Neonatal Medicine 2007; 12, 87-91.
Skinfold Calipers
This is a common method, typically performed using calipers that compress the skin at certain points on the body. While non-invasive, this method suffers from poor accuracy due to variations in fat patterning, misapplication of population specific prediction equations, improper site identification for compressing the skin, poor fold grasping, and the necessity for significant technician training to administer the test properly. Also, no successful methodology for determining infant body composition using skinfold measurement has been devised.
Bioelectrical Impedance (BIA)
BIA measurements rely on the fact that the body contains intracellular and extracellular fluids that conduct electricity by passing a high frequency electric current through the body. BIA determines body composition based on the body’s measured impedance in passing the current and known impedance values for human muscle tissue. However, this method can be greatly affected by the hydration state of the subject and by variations in temperature of both the subject and the surrounding environment. BIA has not been successfully applied with infant subjects. A recent study shows that BIA provides insignificant additional information when compared to anthropometry alone; i.e., infant weight was a better predictor of FFM when compared to the impedance index.
29. Body composition of preterm infants measured during the first months of life: bioelectrical impedance provides insignificant additional information compared to anthropometry alone. Dung NQ, Fusch G, Armbrust S, Jochum F, Fusch C. Confidential - 5 Eur Journl of Pediatrics. 2007; Vol 166 No.3, 215-222.
Dual Energy X-Ray Absorptiometry (DXA)
DXA is a technique that was originally developed for determining bone mineral content in the detection and treatment of osteoporosis. More recently, application of the technique has been expanded to include the analysis of fat and lean mass of soft tissue, in addition to bone mass. In a study that compared DXA to a criterion 4-compartment model in adults, DXA body composition measurements were found to have a bias which varied according to the sex, size, fatness, and disease state of the subject. Variations between software versions (for subjects weighing < 40 kg) and manufacturers have also been reported. Apart from studies questioning the accuracy and validity of DXA in the pediatric population, the testing procedure is affected by the following factors:
- Operator-related issues
Placement of external calibration standard
Placement of blankets
- Subject-related issues
Artifacts due to subject movement
30. DEXA measurements in small subjects: Conditions affecting clinical measurements. Koo WWK, Hockman EM, Hammami M. Journal of American College of Nutrition, 2004 Vol. 23, No. 3, 212-219.