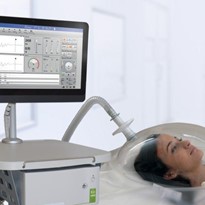
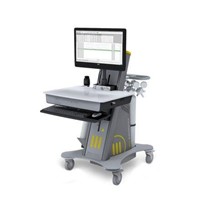
COSMED is glad to announce the U.S. Food and Drug Administration (FDA) clearance of Q-NRG+ and Q-NRG, a metabolic monitoring device utilizing Indirect Calorimetry (IC) technology intended for the measurement of Resting Energy Expenditure (REE) in patients who are mechanically ventilated or spontaneously breathing.
The FDA has granted marketing approval for the device in the US market where we expect to launch the product during the next ASPEN 2020 Nutrition conference taking place in Tampa, FL on March 28-31.
Q-NRG is a result of COSMED’s collaboration with world-class institutes in the field of nutrition support in intensive care units. Product concept and specifications have been designed together with the ICALIC Clinical Trial study group; this collaboration made possible the development of an accurate metabolic system simple to use and, at the same time, able to solve all typical pitfalls of Indirect Calorimetry technology with clear advantages compared to Predictive Equations.















-160x160-state_article-rel-cat.png)






%20(1)-160x160-state_article-rel-cat.png)